Abstract

Introduction Dual translocation of MYC and BCL2 or the dual overexpression of these proteins in patients with aggressive B-cell lymphomas, termed double-hit lymphoma (DHL) and double expressing lymphoma (DEL), respectively, have worse outcomes after standard chemoimmunotherapy with R-CHOP. DA-EPOCH-R has been used as a strategy to overcome chemoresistance. Lenalidomide (LEN) is an immunomodulatory agent with multiple anti-tumor properties including inhibition of the NF-kB pathway, which may enhance the treatment response in MYC-driven lymphomas. This phase I/II study evaluated the safety and efficacy of DA-EPOCH-R with LEN in DHL and DEL. The phase I portion has previously been reported with recommended phase II dose (RP2D) of LEN 15mg daily (day 1-14) per 21 day cycle with DA-EPOCH-R (Godfrey et al. Cancer 2019). Here we report the phase II results.
Methods Adult patients with newly diagnosed DLBCL (stage II-IV) with dual MYC and BCL2 translocations (DHL) or with IHC ≥40% MYC and ≥70% BCL2 (DEL) were treated with LEN (15mg) daily on days 1-14 every 21 days with DA-EPOCH-R for 6 cycles. Patients with complete remission (CR) or partial remission (PR) received either a consolidative auto-SCT (if eligible) or maintenance LEN 10mg daily (days 1-14 every 21 days) for 12 cycles. The primary objective was to determine the 1- and 2-year progression free survival (PFS) with LEN plus DA-EPOCH-R. Secondary objectives included end-of-induction CR and overall response rate, as well as adverse events (AE) by CTCAE v4.0. Intention-to-treat analysis was performed, and response/survival data included phase I patients. Data lock was 7/20/22. Pre-specified primary evaluation of PFS was done with an optimal two-stage design and planned enrollment of at least 46 patients (up to 55). We hypothesized the addition of LEN to DA-EPOCH-R would increase 1-year PFS from 40% (null) to at least 60% (alternative) (α=0.05 and β=0.20). This study was IRB-approved and registered (NCT02213913).
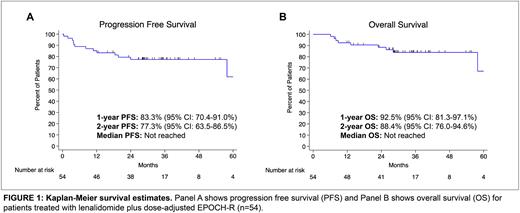
Results 54 patients with median age 65 (25-82) including 15 patients in the phase I portion (12 at RP2D or above) were enrolled and treated between 12/2014 and 1/2020. 48/54 (88.9%) were stage III or IV, and 27/54 (50.0%) had DHL. During induction treatment, 40/54 (74.1%) received all six cycles and 50/54 (92.6%) received at least four cycles. CNS prophylaxis was optional, and all 33/54 (61.1%) received IT methotrexate. The 1- and 2-year PFS were 83.3% (95% CI 70.4-91.0) and 77.3% (95% CI 63.5-86.5), respectively. The 1- and 2-year OS were 92.5% (95% CI 81.3-97.1) and 88.4% (95% CI 76.0-94.6). At median follow up 29.3 months, the median PFS and OS were not reached (Figure 1).
The ORR after LEN and DA-EPOCH-R induction was 49/54 (90.7%) with CR of 46/54 (85.2%). Among responders, 31/49 (63.3%) received maintenance LEN with median 11 cycles (1-12), and 14/31 (45.2%) completed full maintenance. AE during induction was the most common reason for not receiving maintenance (9/18, 50%), and 9/31 (29.0%) stopped maintenance due to an AE. Only 1/49 (2.0%) received an auto-SCT. Of the patients with DHL (n=27), the ORR was 23/27 (85.2%) with CR of 22/27 (81.5%). For patients with DHL (median follow-up 29.2 months), the 1- and 2-year PFS were both 74.1% (95% CI 53.2-86.7), and the 1- and 2-year OS were 88.7% (95% CI 68.8-96.2) and 84.1% (95% CI 62.5-93.7). Median PFS and OS were not reached.
For treatment-related toxicity, 43/54 (79.6%) experienced a grade 3-4 hematologic AE, and 19/54 (35.2%) had grade 3-4 neutropenic fever. 23/54 (42.6%) had any grade infection, and 12/54 (22.2%) were grade 3-4. 10/54 (18.5%) had a thromboembolic event, and 5/54 (9.3%) were grade 3. Peripheral sensory neuropathy was the most common non-hematologic AE with 38/54 (70.4%) any grade and 2/54 (3.7%) grade 3-4. Other common non-hematologic AEs included fatigue (66.7%), constipation (53.7%), and nausea (51.9%) with most being grade 1-2 and only 2-6% grade 3. Two patients (3.7%) had a therapy-related myeloid neoplasm at 6 months and 1.5 years after starting treatment, respectively. There was no treatment-related mortality.
Conclusion LEN with DA-EPOCH-R for patients with DEL and DHL has a high response rate, encouraging survival, and met the primary endpoint for PFS. Considering that the vast majority of relapses are expected in the first 24 months after therapy, these results are promising and warrant further study. The specific benefit attributable to LEN needs to be tested in a randomized setting.
Disclosures
Godfrey:Secura Bio: Research Funding; Merck: Research Funding. Nabhan:Caris Life Sciences: Current Employment. Kline:Karyopharm, Kite/Gilead, Merck, MorphoSys, Seagen, Verastem: Consultancy; iTeos, Merck, Verastem: Research Funding. Bishop:Servier: Speakers Bureau; ADC Therapeutics: Speakers Bureau; Sana Biotechnology: Consultancy; Chimeric Therapeutics: Consultancy; Tmunity: Research Funding; Triumvira: Research Funding; Immatics: Research Funding; Autolus: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau. Stadler:CME providers (sponsorship unknown): Applied Clinical Education, Dava Oncology, Global Academy for Medical Education, OncLive, PeerView, Research to Practice, Vindico: Speakers Bureau; Calico Life Sciences, Caremark/CVS, EMA Wellness: Consultancy; Astra-Zeneca, Bayer, Merck, Pfizer, Treadwell Therapeutics: Other: DSMB; Apotex, DRL, Mylan, Sandoz: Consultancy. Karmali:Eusa: Consultancy; Karyopharm: Consultancy; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Takeda: Research Funding; BMS/Celgene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Other: Advisory Board; Genentech/Roche: Consultancy, Other: Advisory Board; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; AstraZeneca: Other: Advisory Board, Speakers Bureau; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau. Venugopal:BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Smith:Portola: Research Funding; Celgene: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy; Adaptive: Consultancy; Morphosys: Consultancy; BMS: Consultancy; Gilead: Consultancy; ADC Therapeutics: Consultancy; Kite Pharma: Consultancy; Genentech: Consultancy; Bayer: Consultancy; TGTX: Consultancy; Gamida Cell: Consultancy; Bantam: Consultancy; Karyopharm: Consultancy; Chair, Lymphoma Research Foundation SAB: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Lenalidomide in combination with dose-adjusted EPOCH-R
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal